Abstract
Background: Outcomes of adults with ALL has remained poor as compared to pediatric ALL. While biological characteristics of the disease can increase the risk of poor treatment responses and resistance, age and concurrent comorbidities limit patient's tolerability to aggressive therapies and predispose to treatment related adverse effects, ultimately affecting survival. Despite similar chronological age, patients vary widely with respect to comorbidities and other aspects of health such that routine performance assessment scales such as Eastern Cooperative Oncology Group (ECOG) or Karnofsky Performance Scale (KPS) may not be accurate in assessing their fitness for therapy. Several measures such as Hematopoietic Cell Transplantation-Comorbidity Index (HCT-CI), Charlson Comorbidity Index (CCI), Cumulative Illness Rating Scale-Geriatric (CIRS-G) and Adult Comorbidity Evaluation-27 (ACE-27) have been reported in the literature to assess comorbidities in patients receiving intensive treatments, but their utility when it comes specifically to ALL patients is unknown, given the lack of studies assessing role comorbidities in ALL.
Methods: We conducted a retrospective single center analysis of adults (age >=18) with ALL to assess the effect of comorbid conditions using available scales and determined their association with outcomes. Patients with a diagnosis of ALL (B/T-cell) who received first line therapy between 2009-2021 were included. Key exclusion criteria were patients with relapsed/refractory ALL and with incomplete follow-up. Comorbid conditions prior to first line therapy were calculated using four scales - HCT-CI, CCI, CIRS-G and ACE-27. Baseline demographic and disease characteristics, first line treatment, response, outcomes were collected using chart review. Descriptive statistics, logistic regression, Cox Proportional Hazards regression method was used for analysis. Concordance (C-statistic) and 95% confidence interval was reported for each model for comparison. A receiver operating characteristic (ROC) curve was also created for each outcome to compare the performance of base and comorbidity score models.
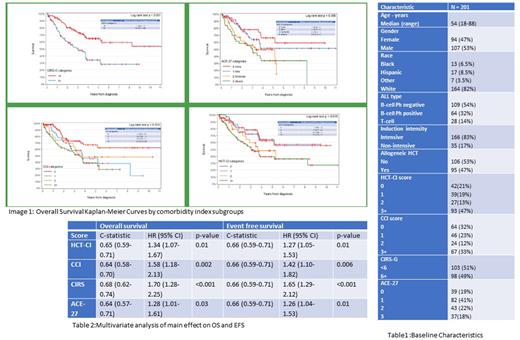
Results: A total of 201 patients were included for analysis. Median age was 54(range 39-65), 53% were males, 82% were Caucasians, 8.5% Hispanics. 86% had B-ALL (32% were Philadelphia chromosome positive), 83% received intensive therapy. Median OS was 3.8 years (3.7-not reached) and Event free survival was 2.0(1.5,3.3) for the entire patient population. Patients were categorized in 0(22%), 1 (19%),2(13%) and 3+(46%) per HCT-CI, 0(32%), 1(23%),2(12%) and 3+(33%) per CCI. In CIRS-G scale patients were categorized in to 2 groups of <6 and >=6(51.5% vs 48.5%). In ACE-27 scale there were 4 categories (none(0),mild(1) moderate(2) and severe(3)) with 19%, 41%, 21% and 18% in each group consecutively. All four comorbidity scales were able to predict overall and event-free survival with statistical significance. Patients with HCT-CI category of <=1 had statistically significant OS and EFS advantage when compared to patients with a score of >=2 (Not reached vs 3.1-3.7 yrs for OS, 2.4-5.2 vs 1.4-1.6 yrs for EFS). Patients with CCI category 0 (not reached, 3.4) had better median OS and PFS than categories 1,2 (3.7 years OS, 1.8-3.1 yrs PFS) and 3+ (3.4, 1.4 yrs). CIRS-G category of <6 score had better median OS and PFS (not reached) than 6+ category (3.0 yrs). In ACE-27 scale, 0 and 1 categories had better OS and PFS (not reached and 3.4 yrs, not reached and 2.3 yrs vs 3.0-3.2 and 1.4 yrs) than moderate and severe (2,3) categories. Though all scales could predict OS and EFS, the overall survival model with CIRS-G scale had a significantly higher C-statistic compared to the base model than other scales. The HCT-CI and CIRS scores were each significant in their models for complete remission, but there were no significant differences between c-statistics among any of the CR models. None of the comorbidity scores were significant in predicting 90-day readmission or ED visit.
Conclusions: Comorbidities as assessed by HCT-CI, CCI, ACE-27 or CIRS-G were significantly associated with survival in adults with ALL. Incorporation of these formal methods of comorbidity assessment in clinical practice is needed to assess ALL patients at baseline and tailor therapy accordingly. Prospective studies to validate and identify the optimal comorbidity determination scale in ALL is needed.
Disclosures
Atallah:BMS: Consultancy, Speakers Bureau; Novartis: Consultancy, Research Funding; Takeda: Research Funding; Abbvie: Consultancy, Research Funding, Speakers Bureau; Blueprint: Speakers Bureau. Michaelis:Jazz Pharmaceuticals: Other: Funding for Research Study to my Institution; Abbvie: Consultancy, Other: Consulting, Advisory Board Meeting; Celgene Corporation: Membership on an entity's Board of Directors or advisory committees, Other: Consulting, Advisory Board Meeting; Sierra Oncology: Membership on an entity's Board of Directors or advisory committees, Other: Consulting, Advisory Board Meeting; Incyte Corporation: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal